[ad_1]
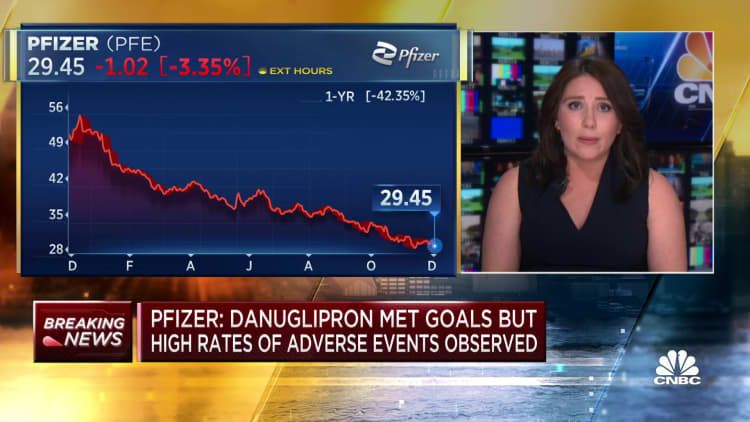
Pfizer on Friday stated it will cease creating the twice-daily model of its experimental weight reduction tablet after overweight sufferers taking the drug misplaced important weight however had bother tolerating the drug in a mid-stage scientific research.
The drugmaker noticed excessive charges of hostile unintended effects, which had been largely gentle and gastrointestinal, amongst sufferers. A major share of sufferers additionally stopped taking the tablet, which goals to be a extra handy different to extremely well-liked weight reduction injections.
“Right now, twice-daily danuglipron formulation is not going to advance into Section 3 research,” the corporate stated.
However Pfizer stated it nonetheless plans to launch knowledge on a once-a-day model of the drug within the first half of 2024, which can “inform a path ahead.” The pharmaceutical big will wait to see that knowledge earlier than deciding whether or not to start out a part three research on the once-daily tablet, which Wall Road views because the extra aggressive type of the therapy.
Shares of Pfizer closed 5% decrease Friday after it introduced the trial outcomes.
Nonetheless, the information on the twice-daily drug is a blow to Pfizer’s hopes to win a $10 billion slice of the booming weight reduction drug market, which CEO Albert Bourla has stated may develop to $90 billion. The corporate is betting on a profitable weight reduction tablet to assist it rebound from plummeting demand for its Covid merchandise and a roughly 40% share value drop this 12 months.
However buyers have been pessimistic about Pfizer’s potential within the weight reduction drug area because the firm scrapped a distinct once-daily tablet in June and proceeded with the much less enticing danuglipron. Now, Friday’s knowledge places Pfizer even additional behind the dominant gamers within the weight reduction drug market, Eli Lilly and Novo Nordisk, that are racing to develop tablet variations of their blockbuster weight reduction and diabetes injections.
Pfizer’s part two trial on its twice-daily tablet adopted round 600 overweight adults who didn’t have Sort 2 diabetes. The trial examined the drug’s impact on weight reduction after 26 or 32 weeks, at completely different dosage quantities starting from 40 milligrams to 200 milligrams.
Like Novo Nordisk’s Wegovy and Ozempic, Pfizer’s tablet works by mimicking a hormone produced within the intestine referred to as GLP-1, which alerts to the mind when an individual is full.
Pfizer stated the trial on danuglipron met the first objective of demonstrating “statistically important” reductions in physique weight.
Sufferers who took the tablet twice a day misplaced 6.9% to 11.7% of their physique weight on common at 32 weeks, and from 4.8% to 9.4% at 26 weeks.
In the meantime, sufferers on a placebo gained 1.4% of their physique weight at 32 weeks and 0.17% at 26 weeks.
When adjusting for the distinction between the load acquire noticed in sufferers who took the placebo, Pfizer’s twice-daily tablet precipitated 8% to 13% weight reduction on common at 32 weeks and 5% to 9.5% at 26 weeks.
The corporate stated excessive charges of hostile occasions had been noticed amongst sufferers within the research, with as much as 73% experiencing nausea, as much as 47% vomiting and as much as 25% experiencing diarrhea. Greater than 50% of sufferers throughout all dose sizes stopped taking the tablet, in comparison with roughly 40% amongst these on the placebo, in response to Pfizer.
No new issues of safety had been noticed, and danuglipron was not related to elevated liver enzymes like Pfizer’s different discontinued weight reduction tablet.
Information from the part two trial will probably be introduced at a future scientific convention or revealed in a peer-reviewed journal.
Wall Road’s expectations
The tolerability points align with some analysts’ predictions forward of the information launch.
Leerink Companions analyst David Risinger wrote in a Monday be aware that the proportion of sufferers who discontinue therapy with Pfizer’s twice-daily danuglipron within the part two trial would possible be greater than those that stopped taking a once-daily tablet from Eli Lilly.
By comparability, 10% to 21% of sufferers who took Eli Lilly’s tablet, orforglipron, in a mid-stage trial discontinued the therapy at 32 weeks as a consequence of hostile unintended effects, he famous.
Risinger stated that is possible as a result of danuglipron’s complete day by day dose is way greater, which can trigger extra hostile results. Sufferers on the best dose dimension of Pfizer’s tablet took 400 milligrams every day, whereas these on the best dosage of Eli Lilly’s drug took 45 milligrams a day.
Pfizer’s phase-two trial additionally did not enable downtitration, or lowering the dose of a drug over time as soon as a particular response has been achieved. Eli Lilly’s mid-stage trial on its tablet did.
Sopa Photographs | Lightrocket | Getty Photographs
There may be hope that sufferers will higher tolerate the once-daily model of danuglipron in comparison with the twice-daily type. Pfizer seems to imagine a once-daily model of the drug may reduce gastrointestinal unintended effects, in response to some analysts.
They pointed to Pfizer’s second-quarter earnings name, when the corporate’s chief scientific officer, Mikael Dolsten, prompt {that a} once-daily model might enhance a affected person’s tolerability of the drug, which may reduce the gastrointestinal unintended effects “which have been seen as limiting” danuglipron.
However Barclays analyst Carter Gould wrote in a Friday be aware that he stays skeptical {that a} once-daily model will “transfer the needle on tolerability given the start line for this dialog.”
He added that it is “more and more obvious the corporate must look to exterior belongings to ship in the marketplace alternative it had portrayed.”
Notably, the load loss brought on by twice-daily danuglipron appeared to fall in need of some analysts’ expectations.
Wall Road was on the lookout for the tablet to point out 14% to fifteen% weight reduction to be aggressive, Cantor Fitzgerald analyst Louise Chen wrote in a Friday be aware. Physicians imagine that 15% weight reduction is “adequate” to persuade them to change from prescribing injectable weight reduction medicine to oral variations, Chen stated.
Leerink’s Risinger additionally wrote in October that Pfizer’s danuglipron wants to point out weight discount within the “mid-teens” percentages to be thought-about aggressive with Eli Lilly’s once-a-day tablet specifically.
Overweight or chubby sufferers who took 45 milligrams of Eli Lilly’s tablet as soon as a day misplaced as much as 14.7% of their physique weight, or 34 kilos, after 36 weeks, in response to the corporate’s phase-two trial outcomes.
Eli Lilly’s outcomes seem according to the load discount brought on by a high-dose oral model of Novo Nordisk’s semaglutide – the lively ingredient used within the diabetes drug Ozempic and weight reduction therapy Wegovy – however came visiting a shorter trial interval.
Each tablets from Eli Lilly and Novo Nordisk seem like “superior to danuglipron at this stage,” TD Cowen analyst Steve Scala stated in a be aware Friday.
“Total, this can be a worse than anticipated consequence for a program that was already taking part in catch-up,” Scala stated of Pfizer’s tablet knowledge.
Greater than 2 in 5 adults have weight problems, in response to the Nationwide Institutes of Well being. About 1 in 11 adults have extreme weight problems.
Clarification: This story was up to date to replicate that some weight-loss knowledge was adjusted to incorporate outcomes from the placebo group.


